CLINICAL TRIALS
A Study to Assess the Efficacy and Safety of Gimlisumab in Subjects With Lung Injury or Acute Respiratory Distress Syndrome Secondary to COVID-19 (BREATHE)
Trial ID: NCT04351243
Efficacy and Safety Study of IV Ravulizumab in Patients With COVID-19 Severe Pneumonia
Trial ID: NCT04369469
PRE-VENT Study in Hospitalized Patients With Severe COVID-19 With or Without Cancer
Trial ID: NCT04404361
Intermediate-size Expanded Access Program (EAP), Mesenchymal Stromal Cells (MSC) for Acute Respiratory Distress Syndrome (ARDS) Due to COVID-19 Infection
Trial ID: NCT04366830
COVID-19 BIOBANKING INITIATIVE
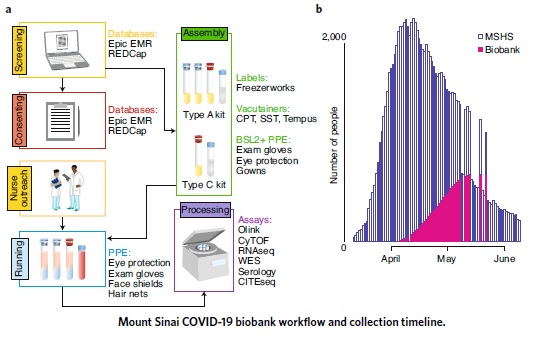 Scientists at the Human Immune Monitoring Center (HIMC), a part of PrIISM, together with the Icahn School of Medicine at Mount Sinai built a COVID-19 research biobank with patients hospitalized at the Mount Sinai health system. The mandate was to design a sample-collection protocol to serve as the backbone for COVID-19 research by yielding high-quality source material for sensitive molecular assays (e.g., RNA sequencing and CyTOF) that cannot be performed on clinical discards. Over a one-month span at the peak of the pandemic, the team enrolled over 700 patients, collected thousands of samples, and have since generated a diverse set of molecular data using state-of-the-art technologies in immunology and genomics. The samples are being analyzed with the goal of identifying biomarkers and therapeutic targets. The collaborative research effort of staff, nurses, doctors, and scientists made this initiative a resounding success. Read more here.
Scientists at the Human Immune Monitoring Center (HIMC), a part of PrIISM, together with the Icahn School of Medicine at Mount Sinai built a COVID-19 research biobank with patients hospitalized at the Mount Sinai health system. The mandate was to design a sample-collection protocol to serve as the backbone for COVID-19 research by yielding high-quality source material for sensitive molecular assays (e.g., RNA sequencing and CyTOF) that cannot be performed on clinical discards. Over a one-month span at the peak of the pandemic, the team enrolled over 700 patients, collected thousands of samples, and have since generated a diverse set of molecular data using state-of-the-art technologies in immunology and genomics. The samples are being analyzed with the goal of identifying biomarkers and therapeutic targets. The collaborative research effort of staff, nurses, doctors, and scientists made this initiative a resounding success. Read more here.
